Technology Shaping the Capital Markets
of the Future
Capital Engine® provides forward-thinking organizations with efficient, scalable private market and investor management solutions, for both traditional and digital assets
REQUEST A DEMONavigate the rough waters of Capital Raising
Built for high-performance capital raising, Capital Engine® provides organizations with a comprehensive, integrated suite of digital investment tools, back office technology and a distribution platform to connect private capital with investors of all types.
Delivered as a service and operated in the cloud, our customized technology solution offers a seamless workflow through the entire investment lifecycle.
Our private markets technology helps organizations leverage the opportunity to better originate and showcase a diverse selection of private investment deals and offer these to investors.Request a call to see our technology in action.
REQUEST A DEMO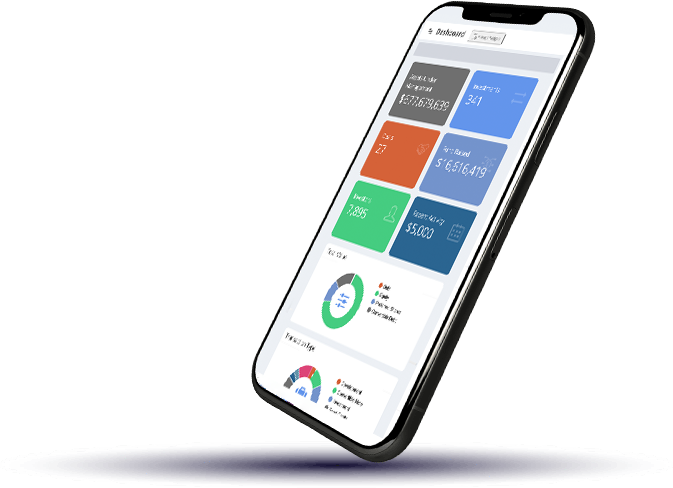
The leading Private Capital and Investor Management solution in the market
Capital Engine® is a fully integrated, end-to-end online system tailored to support the entire investment lifecycle with streamlined workflows for traditional and digital offerings.
Our digital infrastructure automates the entire investment process from investor onboarding, access to virtual deal rooms, and e-signing, to secure online payment, investor reporting and insightful analytics. Our integrated API structure makes syncing with key business technologies easy. Request a call to see our technology in action.
REQUEST A DEMO
Our online Capital Raising and Investor Management tools
Capital Engine® online features include extensive online capital raising and investor management tools to streamline the entire investment process:
Investor Management Tools Regulation & Compliance Infrastructure & Security Fundraising Automation Comprehensive Investor Dashboard Digital Invest Now buttons Integrated API’s with Key Business Technologies
LEARN MORE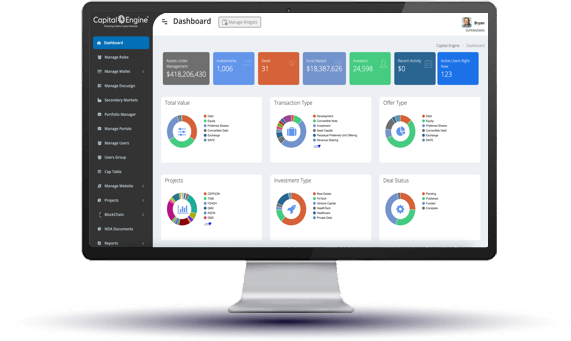
See what Capital Engine® can do for you
Private Label Solution
Customized white label solutions to power private capital, Capital Engine® provides a regulatory compliant branded environment to manage deals and investors for multiple project types and regulations.
Learn More
Strategic Partnership Solution
Strategic partnership solution for investment banks, broker dealers, family offices, wealth managers, accelerators, social impact, real estate funds and Reg CF portals to manage capital raises and investor communities.
Learn More
Capital Engine® Marketplace
Our online marketplace is filling a massive gap in the US market in funding private capital raises and alternative investments. Raise capital with any US regulation, manage investor relations with sophisticated tools, automated compliance, and robust reporting using our proven solution.
Learn More
Our Solutions
Looking For a
Technology Partner For
Your Next Capital Raise?
Fundraising and
managing your
investors just got easier
Apply to Raise CapitalTrusted By Organizations Globally

Eddy Martinez
CRE Income Fund
“ Capital Engine has been a very important part of our process. It's helped us network with potential partners and investors. They also provide access to platforms that help us manage our projects and scale our business. Funding opportunities is an area where Capital Engine really supports us to expand and continue to grow. ”

Nicolas Mugisha
Altvest, Head of Strategy and Special Projects
“ Working with Capital Engine to develop Altvest Securities retirement annuity and insurance platform has been an outstanding experience. Capital Engine truly understood our vision and brought it to life in a way that aligns perfectly with our goals. ”

Mario Romano
Investview Inc., Director of Finance and Public Relations
“ Capital Engine worked with our marketing team to ensure our complete marketing campaign was available in one place for the investor to have access to all the required information. We are extremely pleased with the outcome and the presentation of our offering and successful capital raise of over $8M. ”

Tara Fung
Alto, Chief Revenue Officer
“ Alto and Capital Engine share a mission of providing new ways for startups and entrepreneurs to raise capital and enabling everyday people to take part in investment opportunities, once only available to the wealthy and well-connected. ”

Robert Waitte
CRE Income Fund, Director
“ Capital Engine helped our Texas-based CRE Income Fund fundraise and source new investors more efficiently, all while delivering an unparalleled experience to our existing investors. ”

Carmen Kennison-Brooks
OWN Gold
“ Capital Engine helped OWN Gold source a $10 million SBLC through a Singapore based hedge-fund. ”

Dr. Charlie IN
Raffles Capital, Chairman
“ Capital Engine is liberating every accredited investor to buy pre-equities, safe or bonds or issues. They are giving a lot of investors the opportunity to get into such investments at the basement level, instead of waiting for them at the doorway of a public listing. ”

Michael Markowski
Save Change World
“ Private market assets are going to grow at twice the rate of public assets reaching up to 65 trillion by 2032, according to Bain and Company. That means that Capital Engine is really in the position to lead this whole marketplace. They've already raised $800 million already for companies on their platform. ”
Strategic Partnerships and Key Business Technologies